WASHINGTON — The U.S. Food and Drug Administration issued a warning Monday, saying that counterfeit at-home tests for COVID-19 had been distributed or used in the United States.
In a news release, the FDA explained that “counterfeit COVID-19 tests are tests that are not authorized, cleared, or approved by the FDA for distribution or use in the United States, but are made to look like authorized tests so the users will think they are the real, FDA-authorized test.”
The FDA said that the issue with the counterfeit tests is that there is a risk of false results because the performance of the tests has not been established by the agency. The FDA’s website has a list of authorized at-home over-the-counter tests for COVID-19.
There have been two brands already targeted by counterfeiters. In their news release, the FDA warned that “counterfeit versions of the FDA-authorized Flowflex COVID-19 Antigen Home Tests are being illegally imported and distributed in the United States through unauthorized distributors and resellers who have no connection to ACON Laboratories, Inc.”
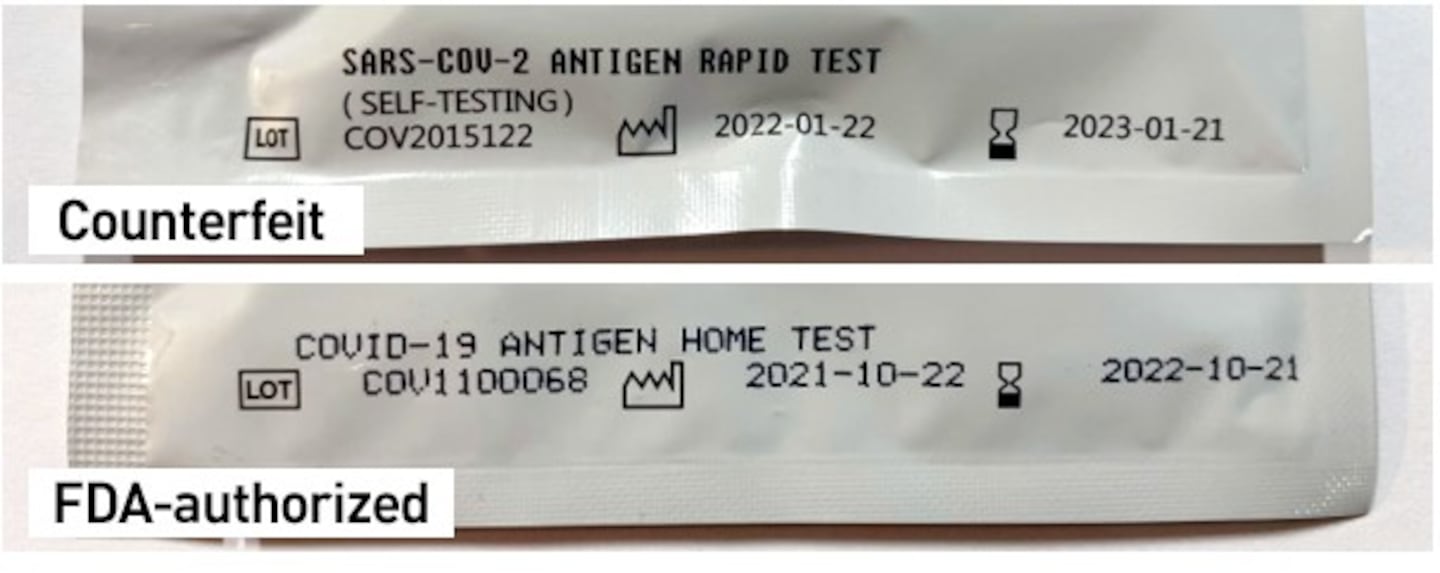
The counterfeit products will not have the lot number/expiration date/2D-datamatrix label that should be found on the test packaging.
The FDA also said that the counterfeit packaging is missing a Spanish-language instruction sheet.
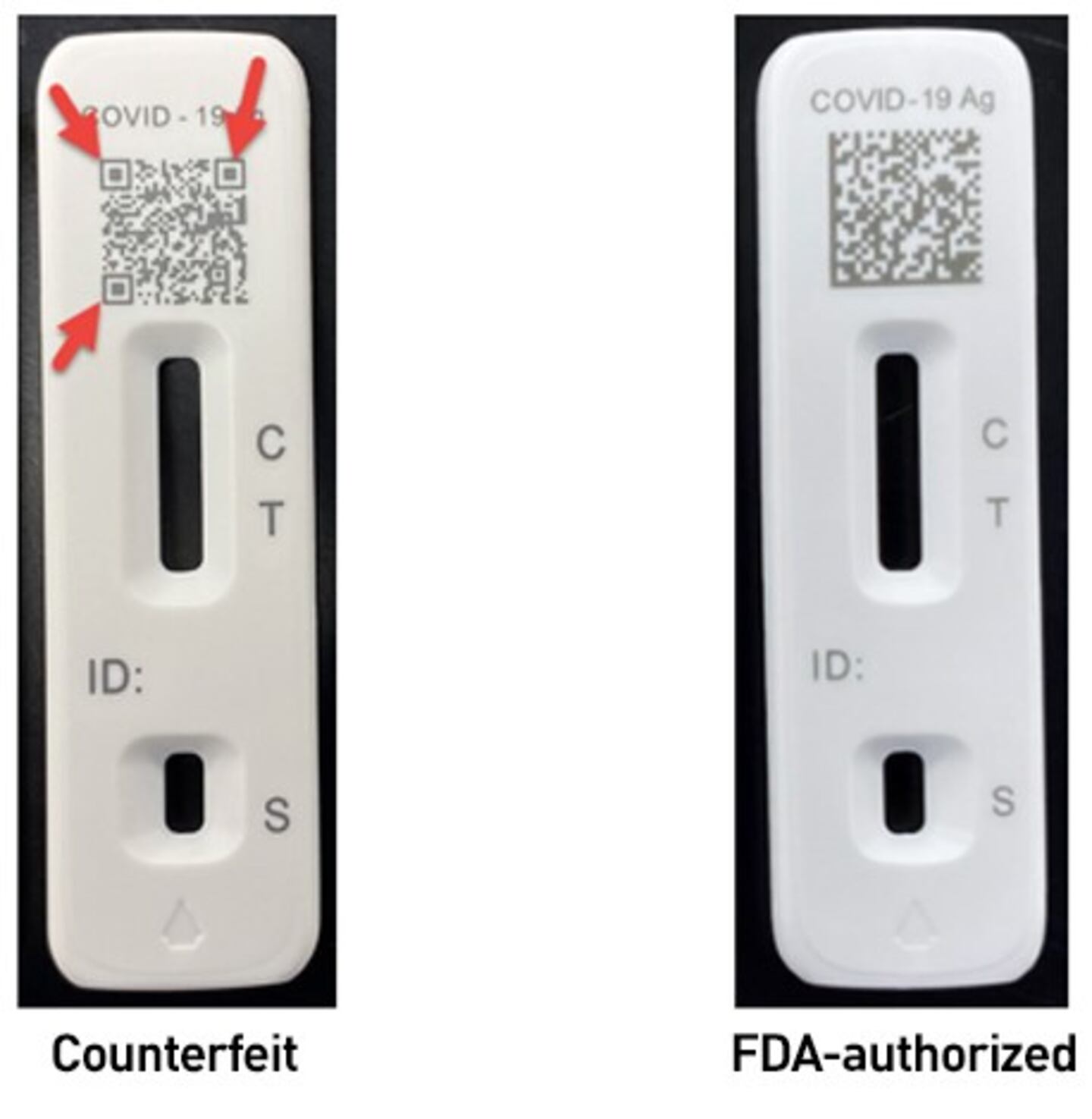
The counterfeit test itself may also look different, with square boxes appearing in the QR code.
The counterfeit FlowFlex tests are separate from the previously announced unauthorized ACON Biotech Flowflex COVID-19 tests. In March, the FDA warned people not to use certain tests packaged in a dark blue box, as we reported at the time.
The FDA said that it had also identified counterfeit iHealth COVID-19 Antigen Rapid Test Kits. The FDA said it is working with iHealth on how to best identify the counterfeit tests, but gave these suggestions of things to look for when determining whether a test is real or counterfeit:
· Poor print quality of images or text on the outside label for the product or in the instructions
· Missing information, such as lot number, expiration date, barcode or QR codes
· Grammatical or spelling errors in product labeling
· Components of kit do not match content description (missing instructions, different number of items than listed)
· Tradename for product differs from authorized labeling found on FDA’s website
The Federal Trade Commission also has tips for staying away from counterfeit tests:
· Check out the seller before you buy, especially if you are buying from a site you don’t know.
· Compare online reviews from a wide variety of websites.
· Pay by credit card, so you can dispute the charge later if necessary.
©2022 Cox Media Group